Product Symbols Glossary - Symbols used on our product, cart and checkout pages on the website.
|
Symbol |
Title of Symbol |
Description of Symbol |
|
|
Perishable |
Item is Perishable |
|
|
Refrigeration Required |
Item requires Refrigeration |
|
|
Freezable |
Item is Freezable |
|
|
HazMat |
HazMat Item - additional shipping charges may apply |
|
|
Nonstock OR Special Order Nonstock and NonReturnable |
Item is NonStock - available to order, typical lead time is approximately 1-2 weeks from time of order placement, unless otherwise stated |
|
|
Country Sales Restriction |
Item has a Country Sales Restriction - see product information below for more details |
 |
Prescription Drug |
This pharmaceutical item is only allowed in select states and territories. See product details for more information. |
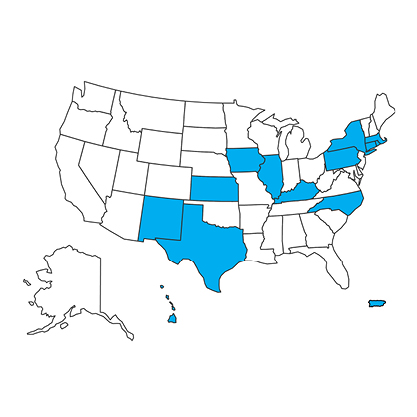 |
State Sales Restriction |
This pharmaceutical item is only allowed in these states and territories. See product details for more information. |
 |
Medical License Required |
This item requires a medical license on file to checkout |
 |
Inventory: Green Status |
This item is currently in stock |
 |
Inventory: Yellow Status |
This item is limited in stock |
 |
Inventory: Red Status |
This item is backordered. See product details for more information. |
Standard: ISO 15223-1, Medical Devices - Symbols to be used with medical device labels, labeling and information to be supplied
|
Symbol |
Symbol Ref. No |
Title of Symbol |
Description of Symbol |
|
|
5.1.1 |
Manufacturer |
Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC. |
|
|
5.1.2 |
Authorized representative in the European Community |
Indicates the authorized representative in the European Community. |
|
|
5.1.3 |
Date of Manufacture |
Indicates the date when the medical device was manufactured. |
|
|
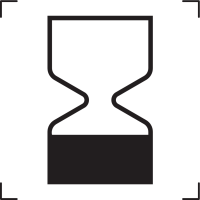
5.1.4 |
Use by date |
Indicates the date after which the medical device is not to be used. |
|
|
5.1.5 |
Batch Code |
Indicates the manufacturer's batch code so that the batch or lot can be identified. |
|
|
5.1.6 |
Catalog Number |
Indicates the manufacturer's catalog number so that the medical device can be identified. |
|
|
5.1.7 |
Serial Number |
Indicates the manufacturer's serial number so that a specific medical device can be identified. |
|
|
5.2.1 |
Sterile |
Indicates a medical device that has been subjected to a sterilization process. |
|
|
5.2.2 |
Sterilized Using Aseptic Processing Techniques |
Indicates a medical device that has been manufactured using accepted aseptic techniques. |
|
|
5.2.4 |
Sterilized using irradiation |
Indicates a medical device that has been sterilized using irradiation. |
|
|
5.2.5 |
Sterilized using steam or dry heat |
Indicates a medical device that has been sterilized using steam or dry heat. |
|
|
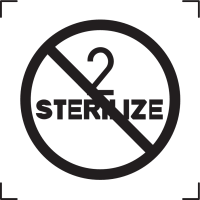
5.2.6 |
Do not re-sterilize |
Indicates a medical device that is not to be re-sterilized. |
|
|
5.2.7 |
Non-sterile |
Indicates a medical device that has not been subjected to a sterilization process. |
|
|
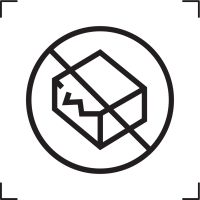
5.2.8 |
Do not use if package is damaged |
Indicates a medical device that should not be used if the package has been damage or opened. |
|
|
5.2.9 |
Sterile Fluid Path |
|
|
|
5.3.1 |
Fragile, handle with care |
Indicates a device that can be broken or damaged if not handled carefully |
|
|
5.3.2 |
Keep away from sunlight |
|
|
|
5.3.4 |
Keep dry |
Indicates a medical device that needs to be protected from moisture. |
|
|
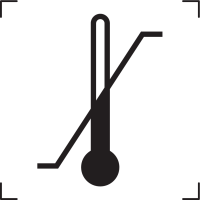
5.3.5 |
Lower limit of temperature |
Indicates the lower limit of temperature to which the medical device can be safely exposed. The temperature is indicated adjacent to the lower horizontal line. |
|
|
5.3.6 |
Upper limit temperature |
Indicates the upper limit of temperature to which the medical device can be safely exposed. The temperature is indicated adjacent to the upper horizontal line. |
|
|
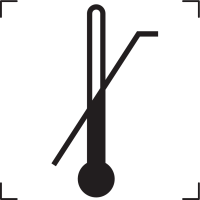
5.3.7 |
Temperature limit |
Indicates the upper and lower limits of temperature to which the medical device can be safely exposed. The temperature is indicated adjacent to the horizontal lines. |
|
|
5.3.8 |
Storage Humidity Range |
Indicates the range of humidity to which the medical device can be safely exposed. |
|
|
5.3.9 |
Atmospheric Pressure |
Indicates the range of atmospheric pressure to which the medical device can be safely exposed. |
|
|
5.4.1 |
Biological risk |
Indicates that there are potential biological risks associated with the medical device. |
|
|
5.4.2 |
Do not re-use |
Indicates a medical device that is intended for one use or for use on a single patient during a single procedure. |
|
|
5.4.3 |
Caution |
|
|
|
5.4.4 |
Caution |
Indicates the need for the user to consult the instructions for use for important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presenting on the medical device itself. |
|
|
5.4.5 |
Contains of presence of natural rubber latex |
Indicates the presence of natural rubber or dry natural rubber latex as a material of construction within the medical device or the packaging of a medical device which may cause allergic reactions. |
|
|
5.5.1 |
In vitro diagnostic medical |
|
|
|
5.5.2 |
Control |
Indicates a control material that is intended to verify the performance characteristics of another medical device. |
|
|
5.5.3 |
Negative Control |
Indicates a control material that is intended to verify the results in the expected negative range. |
|
|
5.5.4 |
Positive Control |
Indicates a control material that is intended to verify the results in the expected negative range. |
|
|
5.5.5 |
Contains Sufficient for tests |
Indicates the total number of IVD tests that can be performed with the IVD. |
|
|
5.5.6 |
For IVD performance evaluation only |
Indicates an IVD device that is intended to be used only for evaluating its performance characteristics before it is placed on the market for medical diagnostic use |
|
|
5.6.2 |
Fluid Path |
Indicates the presence of a fluid path. |
|
|
5.6.3 |
Non-pyrogenic |
Indicates a medical device that is non-pyrogenic. |
|
|
5.6.4 |
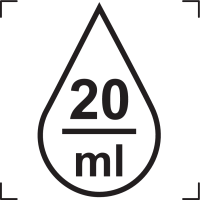
Drops Per Millilitre |
Indicates the number of drops per milliliter. NOTE: The number of drops per milliliter is specified; 20 is shown as an example and should be replaced by the appropriate number of drops per milliliter. |
|
|
5.6.5 |
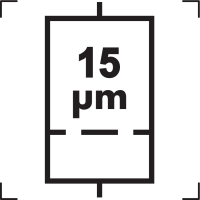
Liquid Filter with Pore Size |
Indicates an infusion or transfusion system of the medical device that contains a filter of a particular nominal pore size. NOTE: The nominal pore size of the filter is specified; 15 is shown as an example and should be replaced by the appropriate pore size. |
|
|
5.7.1 |
Patient Number |
Indicates a unique number associated with an individual patient |
Symbols Not Derived from Standards
|
Symbol |
Symbol Ref. No |
Title of Symbol |
Description of Symbol |
|
|
765/2008/EC 768/2008/EC MDD 93/42/EEC Articles 4,11,12,17, Annex II ) |
Conformité Européene or European Conformity |
Signifies European technical conformity. |
|
|
N/A |
Prescription Only |
Indicates the product is authorized for sale by or on the order of a licensed healthcare practitioner. |